
Unlocking Battery Longevity: Understanding Acid Stratification
Topics: MIXTECH, Battery 101, Transportation, Lead Acid, AGM Batteries, Acid Stratification, Marine Batteries
Topics: Acid Stratification
- Understanding Acid Stratification in Lead-Acid Batteries
- What is Acid Stratification
- How Does Acid Stratification Happen?
- HIgh Deep-Cycle Demands
- Start-Stop Technologies
- Partial State of Charge (PSCOC) Operation
- Effects of Acid STratification
- Uneven Electrolyte Concentration
- Reduced Battery Capacity
- Increased Wear and Tear
- Higher Internal Resistance
- Reduced Cranking Power
- Conclusion
Understanding Acid Stratification in Lead-Acid Batteries
In the world of battery-powered equipment and modern vehicles, maintaining the health of your battery is crucial. One of the biggest threats to battery longevity is acid stratification. Let's explore what it is, how it happens, and its impact on your battery's performance.
What is Acid Stratification?
Acid stratification is a phenomenon that naturally occurs in flooded lead-acid batteries. The electrolyte inside the battery, a mixture of sulfuric acid and water, tends to separate over time. Since acid is heavier than water, it settles at the bottom of the battery's cells, creating a high concentration of acid there, while the upper portion is left with a lower concentration.
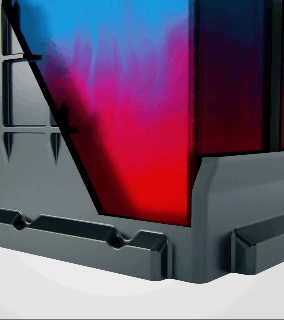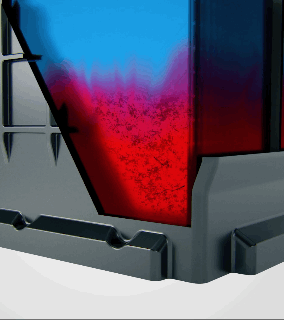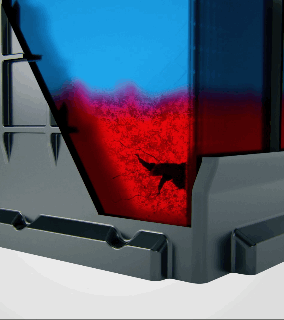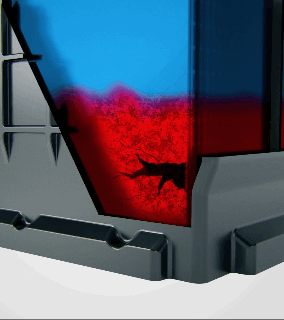
How Does Acid Stratification Happen?
Several factors contribute to acid stratification:
- High Deep-Cycle Demands: Frequent deep discharges and recharges can cause the acid to separate from the water.
- Start-Stop Technologies: The increased cyclic demand from start-stop systems in modern vehicles accelerates this process.
- Partial State of Charge (PSOC) Operation: Regularly operating a battery without fully charging it can lead to stratification.
Effects of Acid Stratification
Uneven Electrolyte Concentration:
-
- The acid sinks to the bottom, leaving the top part of the battery's plates with mostly water. This imbalance reduces the effectiveness of the battery's electrochemical processes.
Reduced Battery Capacity:
-
- The top portion of the plates becomes inactive, reducing the battery's capacity by up to 40% within six months of normal use. This inactive material is often referred to as "dead lead."
Increased Wear and Tear:
-
- The bottom part of the plates works harder, leading to faster wear and tear. Meanwhile, the top part undergoes more charging activity, causing a decline in dynamic charge acceptance (DCA) by 50-70%.
Higher Internal Resistance:
-
- Stratified acid causes higher internal resistance and accelerates sulfation on the lower part of the plates. This results in lower conductivity and reduced battery efficiency.
Reduced Cranking Power:
-
- The increased corrosion from concentrated charging currents at the top of the plates lowers the battery's cranking power, known as Cold Cranking Amps (CCA).
False Charge Readings:
-
- A sulfated battery may give false positive state-of-charge readings. It might appear fully charged but will only provide low CCA and Ampere-Hour (AH) capacity.
- A sulfated battery may give false positive state-of-charge readings. It might appear fully charged but will only provide low CCA and Ampere-Hour (AH) capacity.






