
How Lithium Batteries Work
All batteries work on the principle of moving electrons and ions between two terminals, the cathode (positive) and anode (negative), separated by an electrolyte.
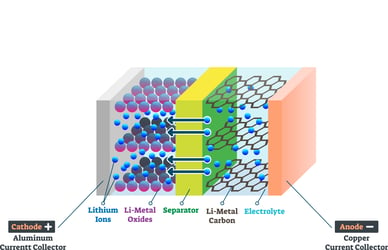
In a rechargeable battery, the anode has excess electrons, making it negatively charged. Conversely, the cathode lacks electrons, making it positively charged. The electrolyte, a barrier between the cathode and the anode, is where ions reside in the battery. When a rechargeable battery is connected to a device, ions in the electrolyte flow from the anode to the cathode, allowing the flow of electrons to power the device. Conversely, when the battery is connected to a power source, as part of the charging cycle, ions in the electrolyte flow from the cathode to the anode, moving the electrons from the cathode to the anode. How many times you can charge the battery is determined by the anode/cathode materials, the quality of the materials, and the frequency of recharging.
A battery accepts a charge when the voltage of the charge source is higher than the battery voltage. When using Alternating Current (AC) from the utility grid or a generator, the power conversion device converts the AC power to Direct Current (DC) to charge the batteries. When Direct Current (DC) from a solar panel or wind turbine is used, the power conversion device steps the voltage up or down to match the battery's voltage. Each type of battery chemistry requires a specific voltage for optimal and safe charging.
Different Chemistries of Lithium Batteries
The materials that make up the cathode, and sometimes the anode, are often used to name the battery.
|
Battery Chemistry |
Description |
|---|---|
|
Lithium Cobalt Oxide (LCO) |
The cathode is made of lithium cobalt oxide, and the anode is carbon. |
|
Lithium Manganese Oxide (LMO) |
The cathode is made of lithium manganese oxide, and the anode is metallic lithium. |
|
Lithium Iron Phosphate (LFP) |
The cathode is made of lithium iron phosphate, and the anode is typically carbon graphite. |
|
Lithium Nickel Manganese Cobalt Oxide (NMC) |
The cathode is made of metal oxides of lithium, nickel, manganese, and cobalt, and the anode is typically made from graphite. |
For each type of lithium battery, the different combinations of materials change the characteristics of the battery, such as charge and discharge rates, energy density, and power density. The different chemistries also affect the battery's thermal stability. Depending on your application, some battery chemistries may need to be avoided. Understanding the different properties of these battery chemistries can help you make decisions.
There are thousands of registered combinations of battery chemistries for rechargeable lithium batteries. Scientists are still finding more and more. They also continue to test every combination of battery chemistries to find the best suited for safe, powerful, long-lasting lithium batteries.
Cell Construction
There are three major types of cells in Lithium batteries: Cylindrical, Prismatic, and Pouch cells.



The first distinguishable difference between these cells is their shape. Cylindrical cells are tubular-shaped. Prismatic cells come in a rectangular-shaped metal box. And Pouch cells come in a thin polymer film.
Each cell type has advantages and disadvantages, making it suitable for specific applications. For example, cylindrical cells are standardized, mass-produced, and used in many common applications such as medical, military, industry, EV, and consumer, making them cheaper than other cell types. Prismatic cells come in a few standardized sizes. They can fit in a thinner profile to utilize the full area inside the battery, which can be an advantage depending on the application. Prismatic cells are commonly used in laptops, EVs, and energy storage solutions, all of which are applications where compact size is important. Pouch cells use light materials, are flexible, and have a high energy density. Pouch cells are used in radio-controlled cars, drones, and ultra-thin laptops, where the pouch's light weight, flexibility, and compactness play an important part in the design.
Smart Batteries
Battery manufacturers can also differentiate their batteries by adding a Battery Management System (BMS), usually offered with higher-quality Lithium batteries.
The BMS includes safety mechanisms that disconnect the battery from the circuit when dangerous conditions, such as high/low voltage, high/low temperature, and high current, are encountered. A smart BMS can restore the connection once the dangerous condition has passed.
Some smart BMS communicate in a closed loop with power conversion equipment. The communicated battery data is used to control the charging process. Information shared by a smart BMS usually includes the battery voltage, state of charge, battery temperature, warnings, and other information. By sharing this information, some charging equipment can dynamically adjust charging parameters to achieve a full charge faster.
Some smart BMS will also log battery data, including the dates, times, and watt/hours of charge and discharge cycles, providing insight into battery performance.
Summary
As Lithium batteries are used in many diverse applications, the more you know, the better decisions you can make when buying a device that uses Lithium batteries. From electric vehicles and laptops to phones, Lithium batteries power anything that requires portable power.
ALWAYS consult experts when deciding which battery best suits your application.






